Recently, Professor Wu Yucheng's research group from the School of Materials Science and Engineering of our university has made the latest progress in the field of electrocatalytic CO2 and nitric acid reduction synthesis of urea. Related research results "Spatial management of CO diffusion on tandem electrode promotes NH2 intermediate formation for efficient urea electrosynthesis "was published in the internationally renowned journalACS Energy Lettersand was chosen as the cover.
Urea is an important source of nitrogen fertilizer in agricultural production, and its industrial production mainly depends on the Bosch-Meier process, which requires harsh reaction conditions such as high temperature and high pressure. The conversion of carbon dioxide and nitrate into high value-added urea through clean electric energy at room temperature and pressure is expected to replace the traditional urea synthesis process, which is of great significance to achieve the strategic goal of carbon neutrality. However, the key challenge of electrocatalytic urea synthesis is that the catalyst's ability to adsorb and activate CO2 and nitrate is weak, resulting in low efficiency and selectivity of urea synthesis.
To this end, Professor Wang Yan and Dr. Zhang Jianfang of Professor Wu Yucheng's research group in our University cooperated with Professor Jingjie Wu of the University of Cincinnati in the United States to construct Cu/ZnO stepped series electrodes for electrocatalytic co-reduction of CO2 and nitrate to produce urea. Through a reasonably designed control experiment combined with in situ spectral analysis, it is proved that CO2 is reduced in the upstream ZnO layer of the series electrode to produce a high concentration of CO, and then spreads to the Cu catalyst layer for C-N coupling reaction. The high concentration of CO not only ensures adequate surface coverage of *CO, but also ensures that the surface coverage of *CO is high enough. Moreover, it can promote the deoxygen-hydrogenation reaction of NO3- to generate *NH2 intermediates. The design of this stepped series electrode can efficiently manage the concentration distribution of *CO and *NH2 intermediates in space, improve the performance of C-N coupling reaction, and provide a new idea for electrocatalytic synthesis of urea.
This research group has carried out a series of researches on the controllable preparation of copper based nanomaterials and the regulation of electrocatalytic CO2 and nitric acid reduction properties. In the previous work, the electrochemical reconfiguration effect of copper based catalysts was deeply investigated, and it was found that abundant grain boundaries, twins, stacking faults and other defects were generated in the original location during the electroreduction process, which could greatly improve the electrocatalytic reaction rate. The prepared Sn or F-doped Cu catalyst was used to electrocatalyze the nitric acid reduction of synthetic ammonia reaction, and obtained the industrial current density of synthetic ammonia, and the Faraday efficiency reached more than 95%. The relevant results were published in the well-known journalsSmall(Small, 2023, 19, 2302295) andMaterials Chemistry Frontier(Materials Chemistry Frontier, 2023, DOI:10.1039/d3qm00114h).
The above research is fully based on national and provincial research platforms such as the National International Science and Technology Cooperation Base for Advanced Energy and Environmental Materials, the Subject Innovation and Introduction Base for Clean Energy New Materials and Technology Universities (111 Plan), the International Science and Technology Cooperation Base for Advanced Nano Energy Materials in Anhui Province, and the Key Laboratory of Advanced Functional Materials and Devices in Anhui Province. The relevant research work has been supported by the National Natural Science Foundation of China, the Key Research and Development Project of Anhui Province, the Natural Science Foundation of Anhui Province, the China Postdoctoral Fund, and the central University Basic Research funds.
Paper link:
1.ACS Energy Letters:
https://pubs.acs.org/doi/full/10.1021/acsenergylett.3c00824
2.Small:
https://onlinelibrary.wiley.com/doi/full/10.1002/smll.202302295
3.Materials Chemistry Frontiers:
https://doi.org/10.1039/D3QM00114H

(Figure 1. Paper cover)
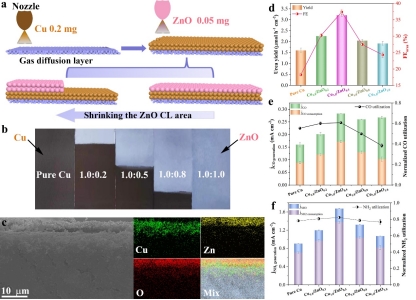
(Figure 2. Structure and urea synthesis performance of stepped Cu/ZnO series electrodes)
(Text:Wang Yan Picture:Zhang Jianfang Examine:Luo Laima)
EDITOR:Zhou Hui
