Our school has made an important progress in the field of regulating reactions with fluorinated compounds.

On August 17,2024,Prof. Huajian Xu and Associate Prof. Jun Xu at Hefei University of Technology published a paper in Nature Communication titled Copper-Catalyzed Highly Switchable Defluoroborylation and Hydrodefluorination of 1-(Trifluoromethyl)Alkynes. This research reported a unified method for the modular synthesis of diverse CF2-containing alkenes from a single starting material.
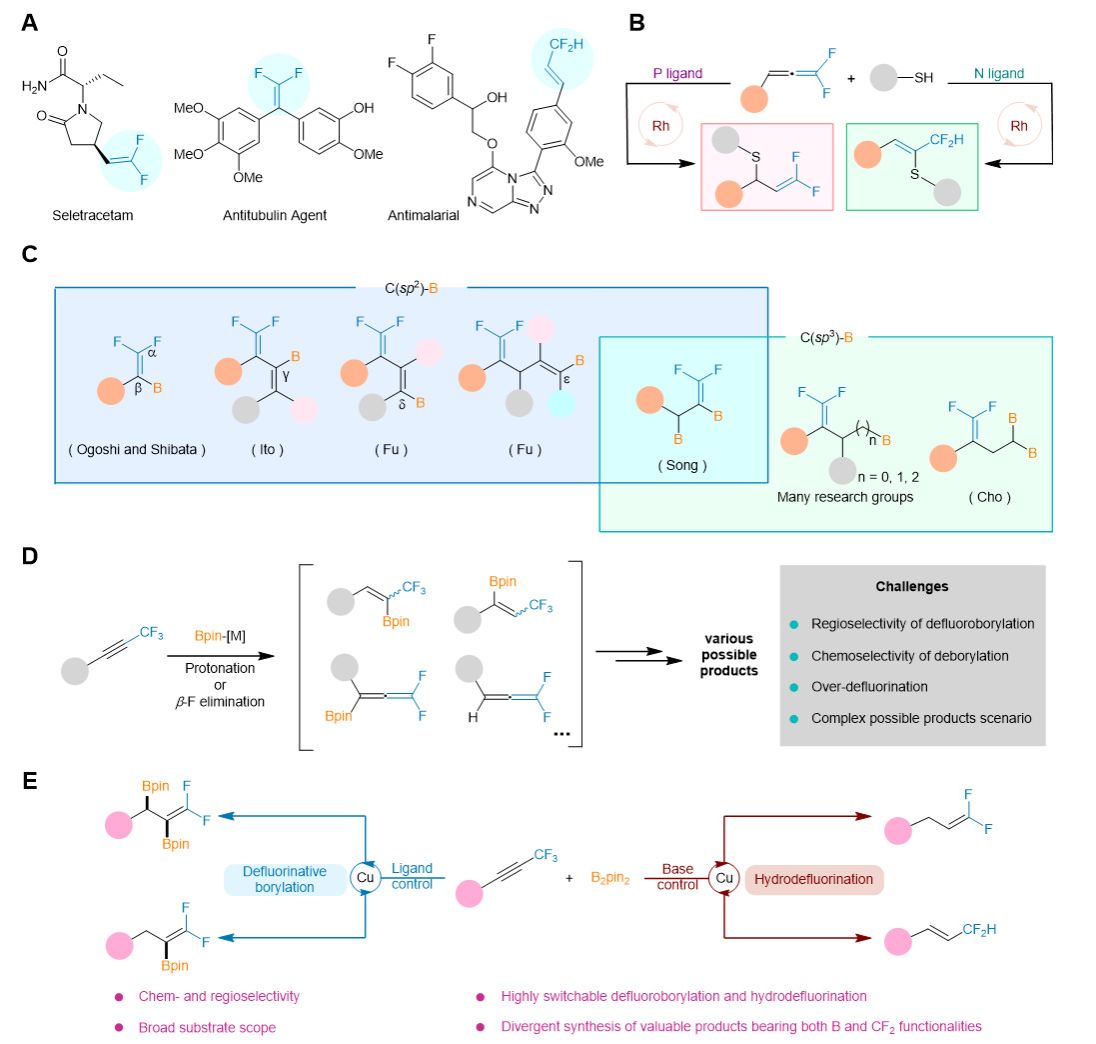
The CF2-containing alkenes, which typically serve as lipophilic isosteric andisopolar synthons to alcohols, amino, thiols or carbonyl groups, play critical roles in pharmaceuticals and drug design (Fig. A). Conventional approaches to access CF2-containing alkenes mainly involve the use of active pre-synthetic difluoromethylating reagents through electrophilic substitution, nucleophilic substitution, radical pathways, or difluorocarbene insertion. However, the modular synthesis of diverse CF2-containing alkenes from a single starting material remains underdeveloped. Only one known method has been identified for the synthesis of these valuable molecules with controllable selectivity: the rhodium-catalyzed regioselective nucleophilic addition of gem-difluoroallenes with thiols, enabling the formation of two types of CF2-containing olefins (Fig. B). Fluorine-containing organoboron compounds have emerged as novel synthons in organic synthesis due to the ease of converting C-B bond into various types of functional groups, which brings diversity and complexity to the target molecules. More recently, a range of elegant and intriguing works on the preparation of gem-difluoroalkenes containing C(sp2)-B bonds, such as β/γ/δ/ε-substituted C-B bonds, have been reported (Fig. C). Although the great progresses have been made, exploiting a unified system for selective construction of fluorinated organoboron skeletons with a controllable number of boronate moieties would present an appealing and highly adaptable strategy for synthesizing these valuable building blocks, potentially paving the way for novel approaches to the synthesis of fluorinated pharmaceutical candidates.
To achieve the above transformation, the authors used trifluoromethylated alkynes and B2pin2 as substrates to achieve chemo- and regio-selectivity by simply varying the solvent, base, and ligand quantity. The primary advantage of this strategy lies in its capability to establish a unified catalytic system that overcomes the complexity and uncertainty faced by alkynes in selectively synthesizing target molecules due to their high reactivity and multiple addition sites ( Fig. 1D).The reaction features readily accessible starting materials, high chem- and regioselectivity, and broad substrate scope with excellent functionality tolerance. This method highlights the potential application of late functionalization of bioactive compounds or drug molecules, and successfully obtained corresponding estrone derivatives in good yields under four standard conditions. In addition, as an important platform, the reaction products can be further converted into diverse fluorinated molecules that are difficult to synthesize using traditional methods.
Experimental data and density functional theory (DFT) calculations elucidate the regioselectivities of Cu-Bpin addition and the regulatory role of the ligand in selective deborylation processes.
Associate Prof. Jun Xu form Hefei University of Technology is the first author of the article. The corresponding authors are Prof. Huajian Xu and Associate Prof. Qi Zhang. School of Food and Biological Engineering at Hefei University of Technology is the first institution of the paper.
Link: https://www.nature.com/articles/s41467-024-51519-y
